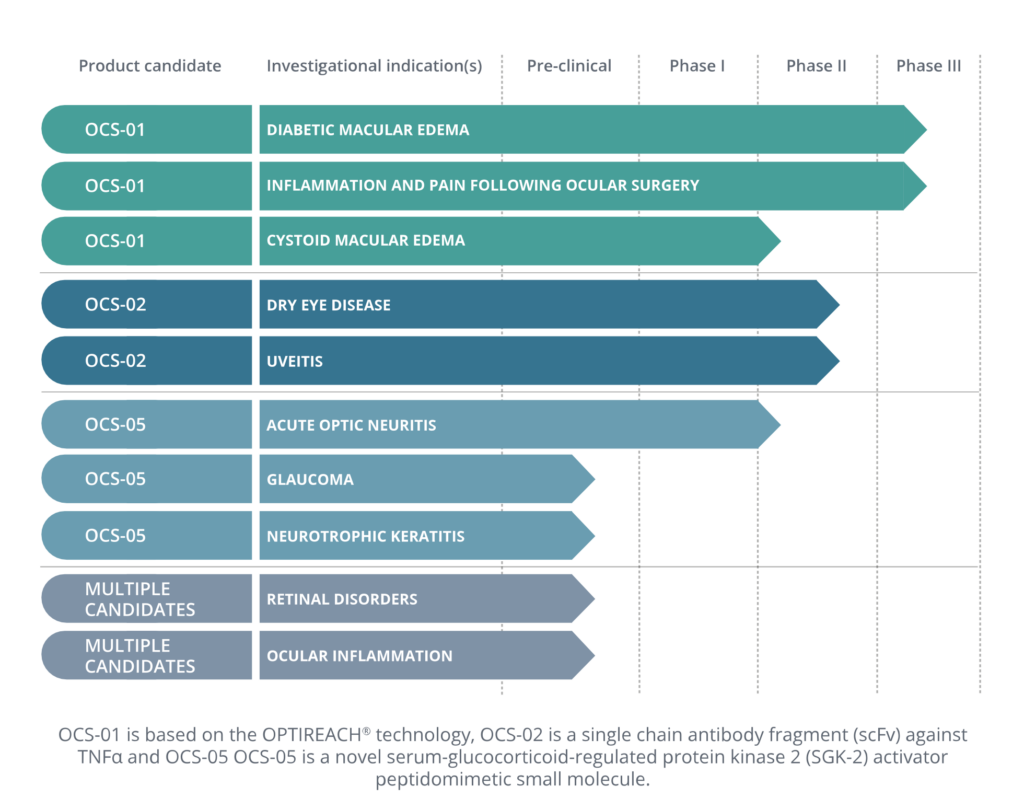
Addressing significant medical needs in key areas of ophthalmology
OCS-01 Eye Drops, A Potentially Transformative Treatment Option for DME
Diabetic Macular Edema (DME), a leading cause of blindness in working-age adults, is estimated to affect around 37 million people worldwide, with a significant number of patients left untreated due to a lack of pre-invasive treatment options.
World-renowned retina experts David S. Boyer, MD, Los Angeles, California, Arshad M. Khanani, MD, Reno, Nevada, and Ramin Tadayoni, MD, PhD, Paris, France, discuss the importance for pre-invasive treatment options which could enable earlier intervention, and the positive Stage 1 results from Oculis’ Phase 3 DIAMOND trial of OCS-01 eye drops in DME.

OCS-01 Eye Drops, A Potentially Transformative Treatment Option for DME

DME Treatment Landscape: Do we need pre-invasive options?

OCS-01 Eye Drops Phase 3 DIAMOND Stage 1 Topline Results

An Investigator’s Perspective on the DIAMOND Stage 1 Trial results
OCS-01 is an investigational drug and has not received regulatory approval for commercial use in any country. Some of the statements in the video reflect experts’ perception of the results from the Stage 1 of the phase 3 DIAMOND clinical trial in DME and some of the forward-looking statements reflect their opinion on potential future use of OCS-01 based on their clinical experience.
OCS-02, A TOPICAL BIOLOGIC anti-TNFα TO TREAT OCULAR INFLAMMATION
Licaminlimab (OCS-02) is a next-generation biologic eye drop treatment in development for both DED and non-infectious anterior uveitis. It is expected that OCS-02’s anti-TNFα mechanism of action and topical route of administration can benefit patients suffering from dry eye, many of whom do not receive adequate relief with current options, as well as patients suffering from non-infectious anterior uveitis and for whom OCS-02 can be a powerful option with a significantly lighter toxicity profile than current options.
Differentiating OCS-02 is the topical delivery of an anti-TNFα construct at increased concentrations. The anti-inflammatory and anti-apoptotic properties of therapeutics inhibiting TNFα activity are well established with anti-TNF pharmaceuticals already approved as systemic treatments for many diseases. In addition, Oculis is advancing the development of OCS-02 in conjunction with the development of a potentially novel genetic biomarker intended to identify patients who may have a greater response to OCS-02 therapy.
Two Phase 2 clinical trials in patients with symptoms of DED were conducted (the first with the predecessor of OCS-02, and the second with OCS-02), as well one Phase 2 clinical trial in acute anterior uveitis. Topical ocular administration of OCS-02 was associated with improvements in the global ocular discomfort score versus vehicle in patients with DED, and with reaching a pre-specified responder rate in patients with non-infectious anterior uveitis, as well as being well tolerated in all three studies. Oculis has initiated the Phase 2b RELIEF trial evaluating the potential of Licaminlimab in signs and symptoms of DED and is preparing to commence another Phase 2b trial in non-infectious anterior uveitis.
OCS-05, A POTENTIAL DISEASE MODIFYING CANDIDATE FOR NEURO-OPHTHA DISORDERS (OR DISEASES)
OCS-05, a novel serum/glucocorticoid-regulated protein kinase 2 (SGK2) activator peptidomimetic small molecule, is in development as a potential disease modifying neuroprotective agent against neurological damage to the optic nerve. Oculis is initially developing OCS-05 as a potential therapeutic to treat AON, a rare disease with high medical need as currently, there is no treatment which is approved by the FDA or the European Commission for AON. OCS-05 has been granted Orphan Drug Designation by both the FDA and the European Commission for this indication.
Pre-clinical studies suggest that OCS-05 is neuroprotective and has remyelinating activity. A UK Phase 1 clinical trial (with 48 healthy volunteers) showed that OCS-05 was well tolerated with good pharmacokinetics (PK) correlation with its pre-clinical animal studies. The results of these studies have enabled the advancement of OCS-05 into a First-in-Patient clinical proof-of-concept trial in France. The Acute OptiC NeUrITis of DemYelinating Origin (ACUITY) trial, a randomized, double-masked, placebo controlled, multiple center trial, is enrolling patients diagnosed with AON.
Should the clinical results of the AON trial prove sufficiently compelling, Oculis intents to evaluate the promise of OCS-05 to treat other neuro-ophthalmic disorders such as geographic atrophy, glaucoma, diabetic retinopathy, and neurotrophic keratitis.

