What is a cataract?
A cataract is a clouding of the lens in the eye that affects vision. Most cataracts are related to aging. Cataracts are very common in older people.
By age 80, more than half of all Americans either have a cataract or have had cataract surgery.
A cataract can occur in either or both eyes. It cannot spread from one eye to the other.
How is it treated?
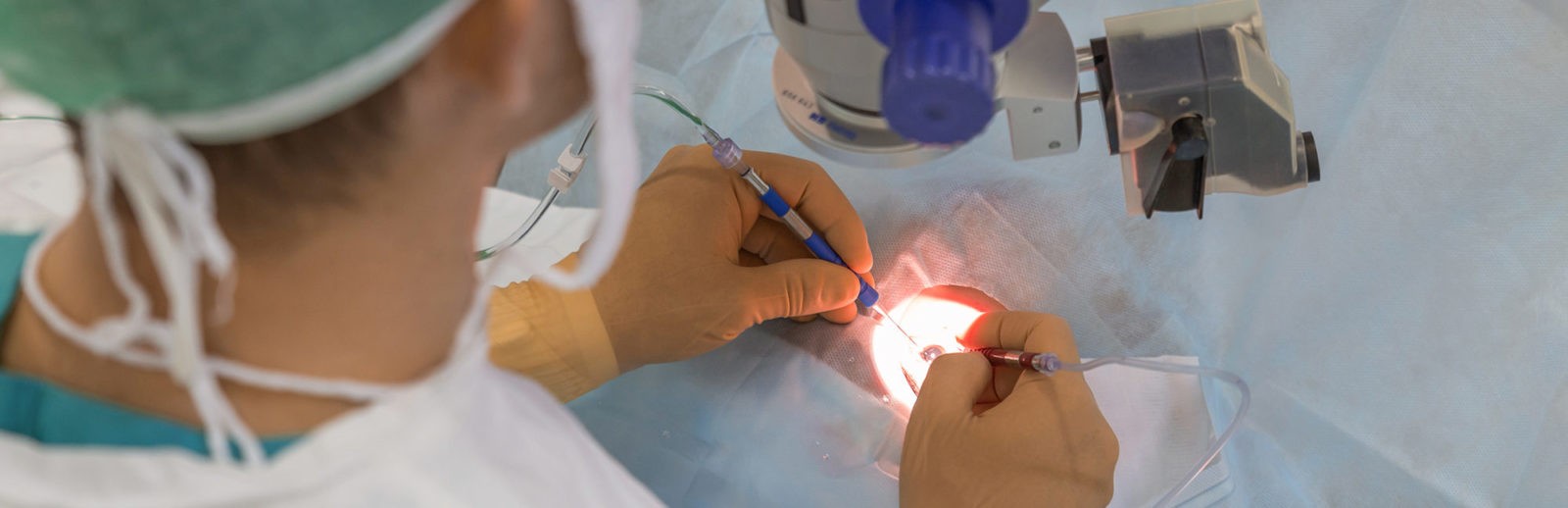
Cataract surgery is an operation to remove your eye’s lens when it is cloudy.
The purpose of your lens is to bend (refract) light rays that come into the eye to help you see. Your own lens should be clear, but with a cataract it is cloudy. Having a cataract can be like looking through a foggy or dusty car windshield. Things may look blurry, hazy or less colorful.
The only way to remove a cataract is with surgery. Your ophthalmologist will recommend removing a cataract when it keeps you from doing things you want or need to do.
During cataract surgery, your cloudy natural lens is removed and replaced with a clear artificial lens. That lens is called an intraocular lens (IOL). Your ophthalmologist will talk with you about IOLs and how they work.
https://www.aao.org/eye-health/diseases/what-is-cataract-surgery
What Causes Inflammation After Cataract Surgery?
Any type of surgery creates an incision, and this promotes release of inflammatory factors. Cataract surgery, while the incision is very small, creates inflammation in the cornea, anterior chamber, and iris. Also, microscopic remnants of the cataract are often left in the anterior chamber of the eye, which lead to continued release of inflammatory factors. Topical anti-inflammatory agents are beneficial at removing all the inflammatory factors and clearing the vision, thus they are very important to use after any intraocular surgery.
https://www.aao.org/eye-health/ask-ophthalmologist-q/what-causes-in?ammation-after-cataract-surgery
OCS-01: A potential once daily treatment for Post-cataract Surgery Inflammation
OCS-01, the first investigational eye drop designed for front- and back-of-the-eye, met both primary endpoints in the Phase 3 OPTIMIZE trial with a once daily regimen for the treatment of inflammation and pain following cataract surgery. The trial results showed OCS-01’s superiority in reducing inflammation and pain vs. vehicle as well as a favorable safety profile. The data is highly consistent with the phase 2 SKYGGN trial for the same indication.
The OPTIMIZE Phase 3 results follow the positive and statistically significant top line results from stage 1 of the Phase 3 DIAMOND trial in Diabetic Macular Edema (DME) reported in May 2023, further highlighting the product’s potential for treating front- and back-of-the-eye diseases.
OCS-01 has been developed using the OPTIREACH solubilizing technology, a proprietary platform that enables the formulation of drugs as non-invasive topical treatments, a longer residence time on the eye surface and enhances their bioavailability in the relevant eye tissues.
If approved, OCS-01 has the potential to become a new standard of care as the first once-daily, topical, preservative-free corticosteroid for treating inflammation and pain following ocular surgery.
To learn more about the OPTIMIZE Phase 3 Results of once daily OCS-01 for the treatment of Inflammation and Pain following cataract surgery, please click here

