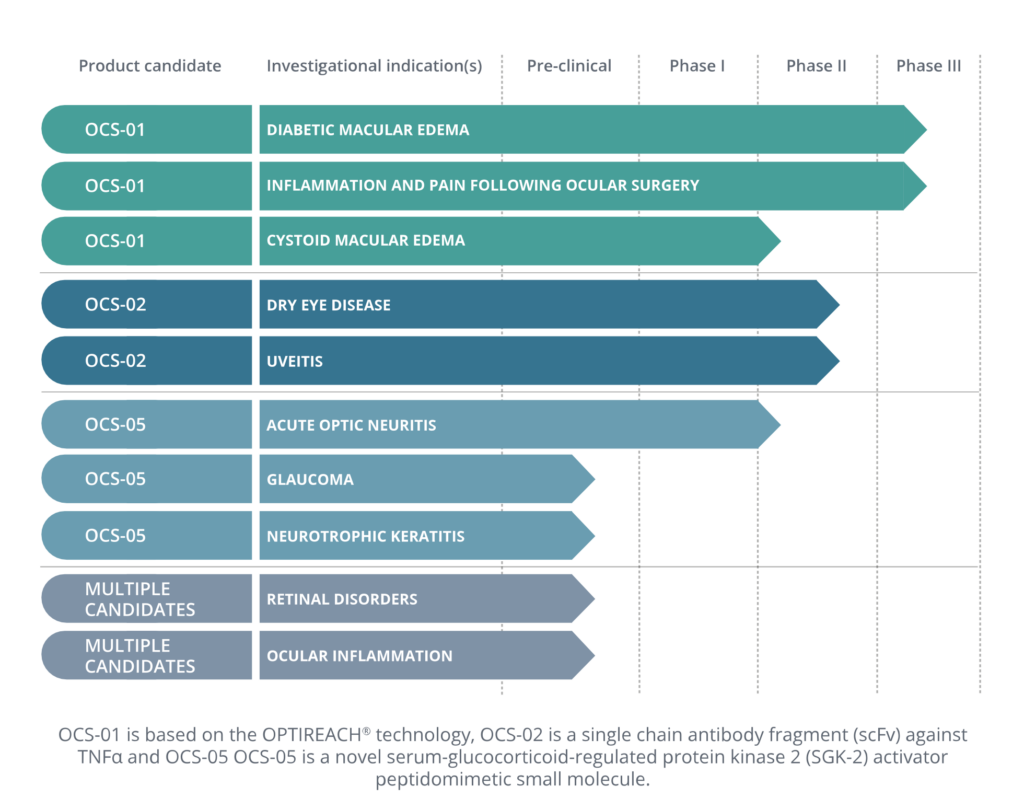
Addressing significant medical needs in key areas of ophthalmology
OCS-01 Eye Drops, A Potentially Transformative Treatment Option for DME
Diabetic Macular Edema (DME), a leading cause of blindness in working-age adults, is estimated to affect around 37 million people worldwide, with a significant number of patients left untreated due to a lack of pre-invasive treatment options.
World-renowned retina experts David S. Boyer, MD, Los Angeles, California, Arshad M. Khanani, MD, Reno, Nevada, and Ramin Tadayoni, MD, PhD, Paris, France, discuss the importance for pre-invasive treatment options which could enable earlier intervention, and the positive Stage 1 results from Oculis’ Phase 3 DIAMOND trial of OCS-01 eye drops in DME.

OCS-01 Eye Drops, A Potentially Transformative Treatment Option for DME

DME Treatment Landscape: Do we need pre-invasive options?

OCS-01 Eye Drops Phase 3 DIAMOND Stage 1 Topline Results

An Investigator’s Perspective on the DIAMOND Stage 1 Trial results
OCS-01 is an investigational drug and has not received regulatory approval for commercial use in any country. Some of the statements in the video reflect experts’ perception of the results from the Stage 1 of the phase 3 DIAMOND clinical trial in DME and some of the forward-looking statements reflect their opinion on potential future use of OCS-01 based on their clinical experience.
OCS-02, A TOPICAL BIOLOGIC anti-TNFα TO TREAT OCULAR INFLAMMATION
Licaminlimab (OCS-02) is a next-generation biologic eye drop treatment in development for both dry eye disease (DED) and non-infectious anterior uveitis. It is expected that licaminlimab (OCS-02)’s anti-TNFα dual mechanism of action, anti-inflammatory and anti-apoptosis, and topical route of administration can benefit patients suffering from dry eye, many of whom do not receive adequate relief with current options, as well as patients suffering from non-infectious anterior uveitis and for whom licaminlimab (OCS-02) can be a targeted option that is tolerated and efficacious.
Licaminlimab (OCS-02) is based on a proprietary single-chain antibody fragment technology specifically designed for topical delivery. The anti-inflammatory and anti-apoptotic properties of therapeutics inhibiting TNFα activity are well established with anti-TNF pharmaceuticals already approved as systemic treatments for many diseases. In addition, Oculis is advancing the development of licaminlimab (OCS-02) in conjunction with the development of a potentially novel genetic biomarker intended to identify patients who may have a greater response to licaminlimab (OCS-02) therapy.
Two Phase 2 clinical trials in patients with symptoms and one Phase 2b in signs of DED were conducted (the first trial in symptoms with the predecessor of OCS-02, and the other two trials with licaminlimab (OCS-02)). Topical ocular administration of licaminlimab (OCS-02) was associated with improvements in symptoms (global ocular discomfort score) and in multiple signs (e.g. inferior corneal fluorescein staining) in patients with DED. Furthermore, licaminlimab was well-tolerated similar to vehicle. In two of the Phase 2 trials, a pre-specified analysis was conducted in patients with a specific TNFR1-related genotype. A more pronounced effect was observed on signs and symptoms of DED with licaminlimab (OCS-02) in this genotype population, showing its potential to be the first precision medicine approach for the treatment of DED.
Licaminlimab (OCS-02) is an investigational drug and has not received regulatory approval for commercial use in any country.
OCS-05, A POTENTIAL DISEASE MODIFYING CANDIDATE FOR NEURO-OPHTHA DISORDERS (OR DISEASES)
OCS-05, a novel serum/glucocorticoid-regulated protein kinase 2 (SGK2) activator peptidomimetic small molecule, is in development as a potential disease modifying neuroprotective agent against neurological damage to the optic nerve. Oculis is initially developing OCS-05 as a potential therapeutic to treat AON, a rare disease with high medical need as currently, there is no treatment which is approved by the FDA or the European Commission for AON. OCS-05 has been granted Orphan Drug Designation by both the FDA and the European Commission for this indication.
Pre-clinical studies suggest that OCS-05 is neuroprotective and has remyelinating activity. A UK Phase 1 clinical trial (with 48 healthy volunteers) showed that OCS-05 was well tolerated with good pharmacokinetics (PK) correlation with its pre-clinical animal studies. The results of these studies have enabled the advancement of OCS-05 into a First-in-Patient clinical proof-of-concept trial in France. The Acute OptiC NeUrITis of DemYelinating Origin (ACUITY) trial, a randomized, double-masked, placebo controlled, multiple center trial, is enrolling patients diagnosed with AON.
Should the clinical results of the AON trial prove sufficiently compelling, Oculis intents to evaluate the promise of OCS-05 to treat other neuro-ophthalmic disorders such as geographic atrophy, glaucoma, diabetic retinopathy, and neurotrophic keratitis.

