Science
Our relentless focus on understanding and addressing unmet medical needs has resulted in transformative innovations that aim to make a real difference in patients’ lives


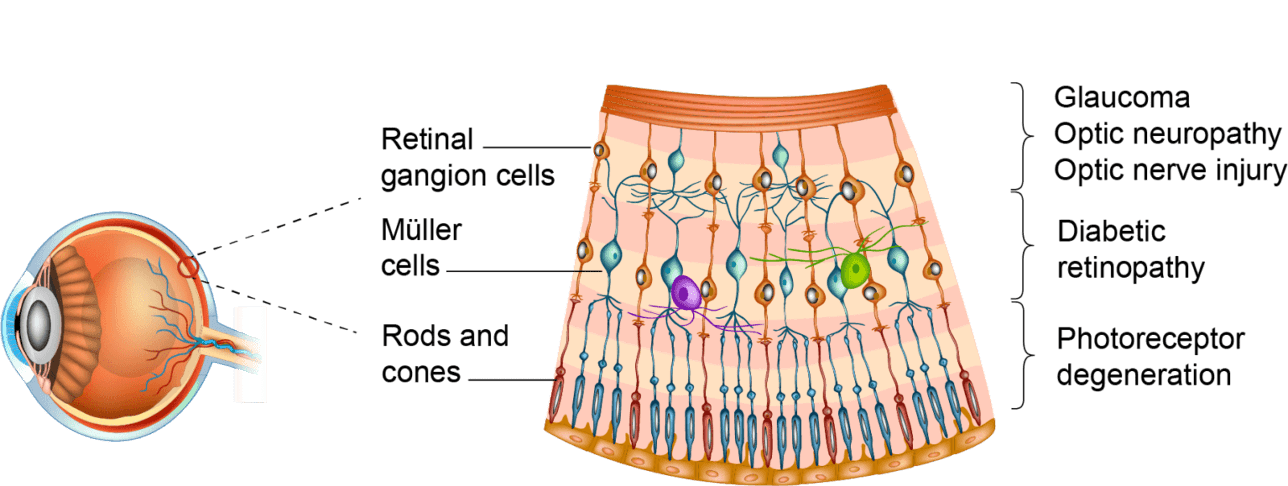
A novel neuroprotective approach to treat optic neuropathies and retinal diseases
OPTIC NEUROPATHIES AND RETINAL NEURODEGENERATION DISEASES SHARE COMMON FEATURES
because the cellular and molecular responses to neurodegeneration (inflammation, oxidative stress, apoptosis) are similar.1
THE AIM OF NEUROPROTECTION
is to increase retinal neuronal survival and preserve visual function, to fulfil unmet medical needs across multiple retinal diseases and optic neuropathies.
Privosegtor is a novel peptoid small molecule that penetrates the blood brain and retinal barrier, with the potential to become a neuroprotective therapy for optic neuropathies and other neuro-ophthalmic diseases.
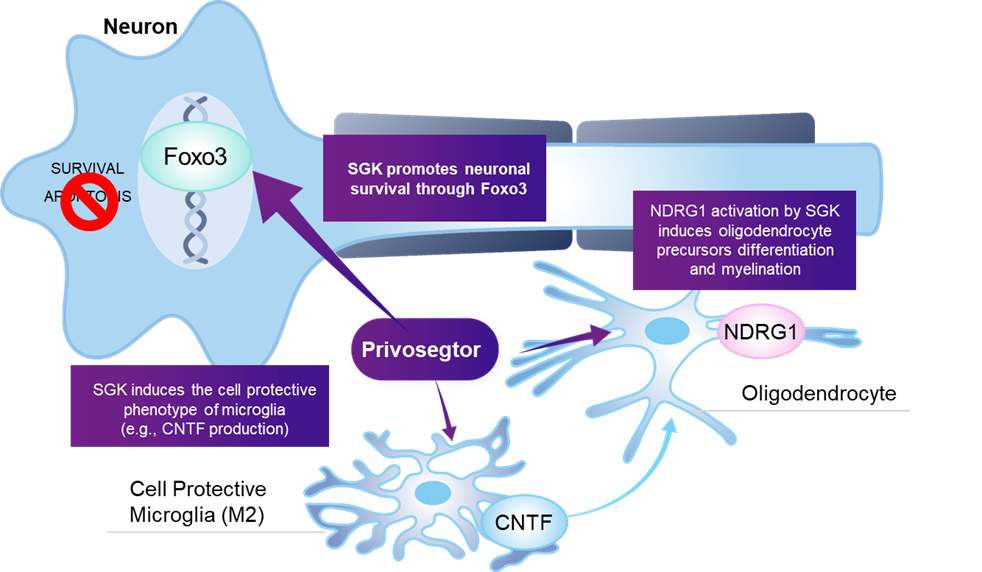
Privosegtor was selected by high-throughput screening (HTS) for neuroprotective properties, confirmed in vivo in glaucoma, multiple sclerosis, and acute optic neuritis models.
The data from in vitro studies also suggest that it modulates the FOXO3 pathway by interacting with the serum–glucocorticoid kinase (SGK) supporting neuronal survival and preservation.
Privosegtor
is being developed as a potential neuroprotective drug to protect neurons affected in neuro-ophthalmic diseases.
Explore Privosegtor as a potential neuroprotective treatment for acute optic neuritis and non-arteritic anterior ischemic optic neuropathy (NAION).
Enabling eye drops to reach the back of the eye
OPTIREACH® is a solubilizing formulation technology that has been developed by Oculis’ Icelandic co-founders Professors Einar Stefánsson, and Thorsteinn Loftsson to enable eye drops to reach the back of the eye. The technology enables:
Increased solubility of drugs in eye drop formulations
Longer residence time on the eye surface
Drug passage to the posterior segment of the eye
Conceptual illustration
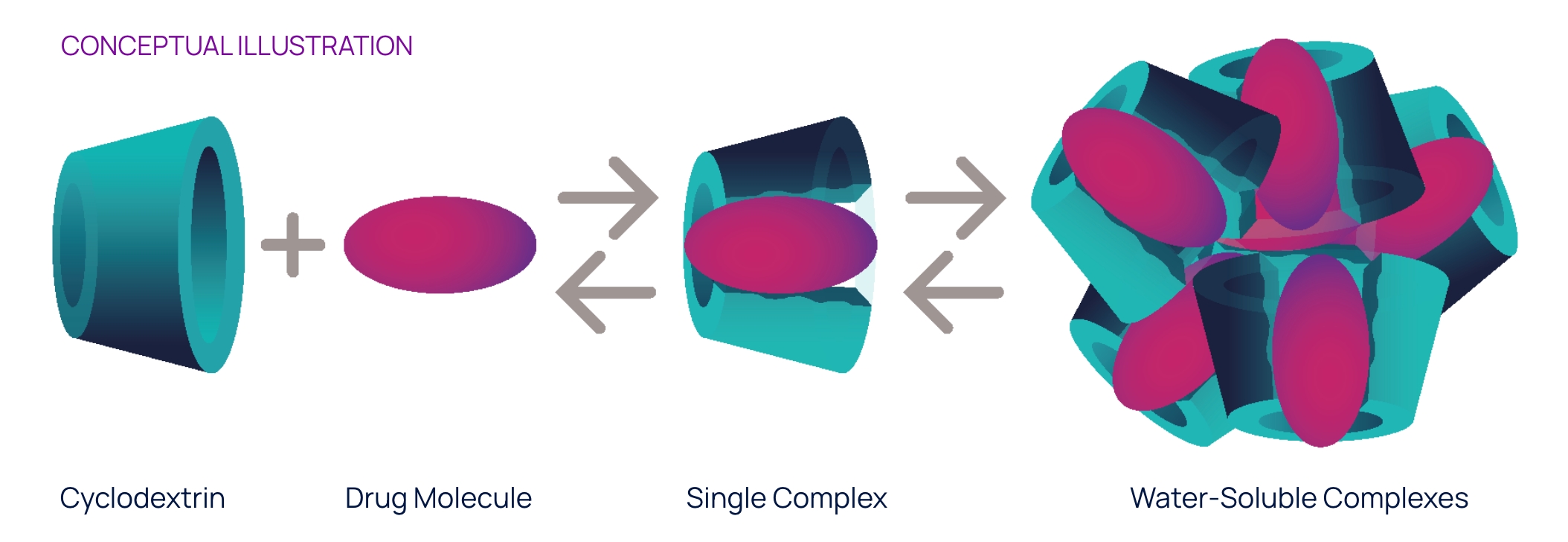
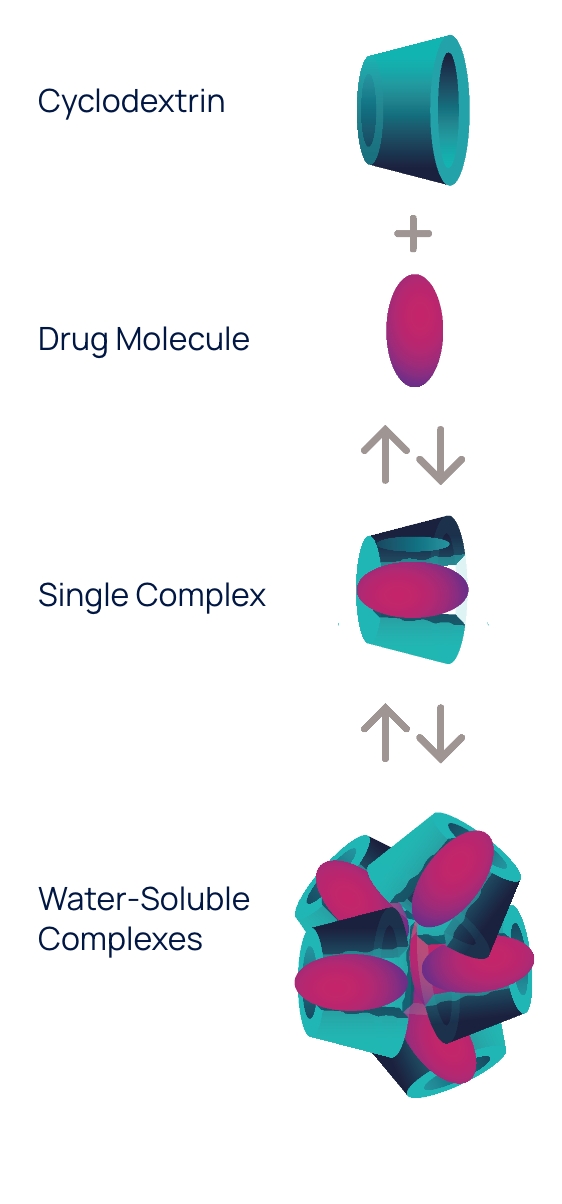
OPTIREACH® improves the ability to formulate drugs as eye drops and enhances their bioavailability in eye tissues
The OPTIREACH® solubilizing formulation technology leverages the unique features of specific cyclodextrins to create stable formulations containing water-soluble drug/cyclodextrin complexes.
The goal of this novel formulation approach incorporating selected features of specific cyclodextrins is to improve the solubility of lipophilic drugs, increase their residence time on the eye surface while enabling the drug passage to the posterior segment of the eye.
The OPTIREACH® solubilizing formulation technology is one of the proprietary platforms underpinning Oculis’ pipeline. It was leveraged to develop Oculis’ most advanced product candidate, OCS-01, an innovative preservative-free eye drop candidate, currently in Phase 3 development in patients with diabetic macular edema (DME).
The goal of this novel formulation approach incorporating selected features of specific cyclodextrins is to improve the solubility of lipophilic drugs, increase their residence time on the eye surface while enabling the drug passage to the posterior segment of the eye.
Pre-clinical and clinical data suggest that the OPTIREACH® solubilizing formulation technology can open the way to formulating drugs as effective and well-tolerated topical treatments of retinal conditions with high unmet need, such as Diabetic Macula Edema. Significant improvements of dosing frequency and tolerability for anterior eye conditions such as ocular inflammation and pain after ocular surgery may also be expected.
OCS-01
has been developed using the OPTIREACH® solubilizing formulation technology
Explore how OCS-01 could become the first non-invasive eye drop to treat DME.
Innovative antibody fragment technology enabling the development of potentially the first anti-TNFα biologic eye drop to treat inflammatory eye diseases
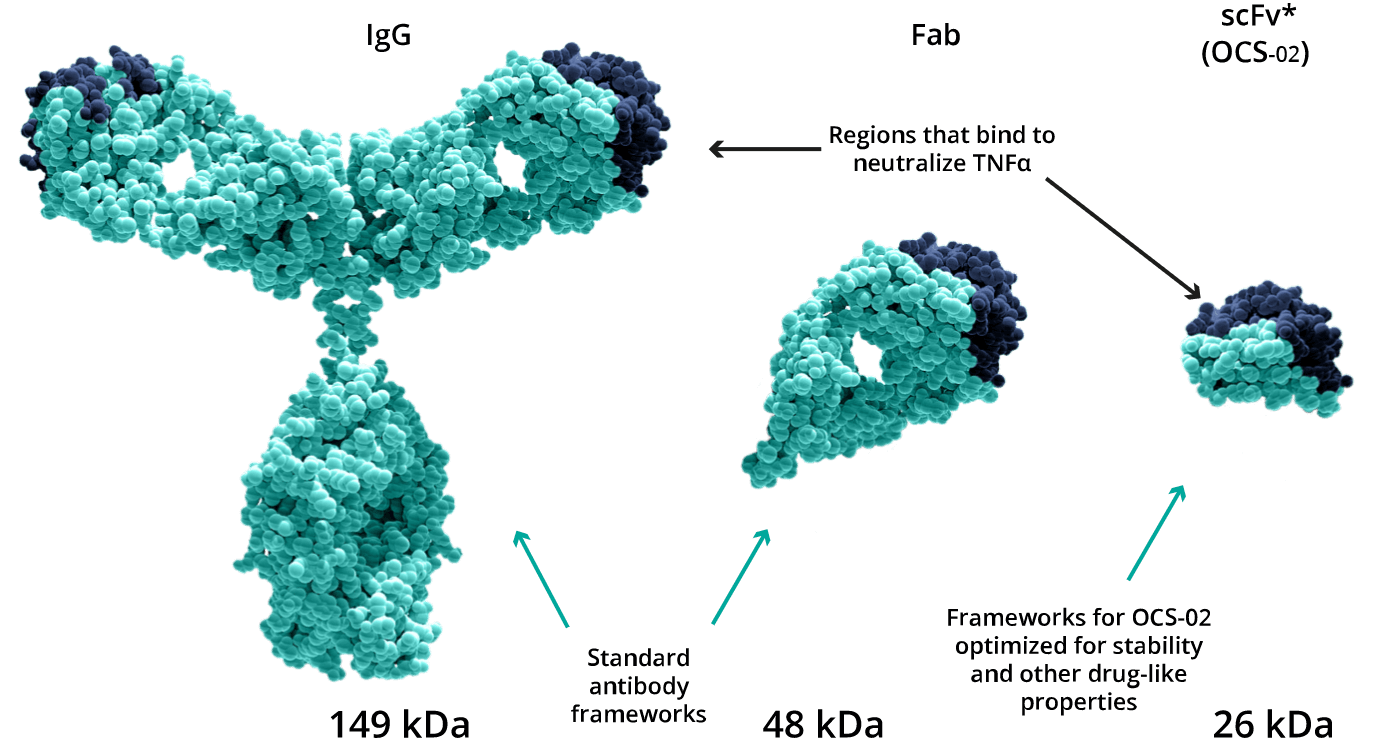
The unique antibody fragment technology allows for the development of a small, pharmacologically active, humanized single chain variable fragment antibody (scFv). The lower molecular weight of the fragment relative to the whole antibody enables the development of a higher formulation concentration enhancing its penetration into ocular tissues. These characteristics make scFv more suitable than full-length antibodies for the topical treatment of ocular surface diseases, such as dry eye disease (DED).
Licaminlimab is an anti-TNFα antibody fragment formulation which binds to and neutralizes the activity of human TNFα. It has the potential to become the first anti-TNFα biologic eye drop to treat ocular inflammation.
CLINICALLY PROVEN MODE OF ACTION (MOA)
With their proven anti-inflammatory and anti-apoptotic dual mechanism of action, anti-TNFα agents have been approved as systemic treatments for several inflammatory diseases and with transformative impact on other therapeutic areas.
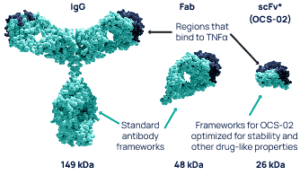
ENHANCED OCULAR PENETRATION
Lower molecular weight allows for higher concentration and enhanced ocular tissue penetration.
PROPRIETARY GENETIC BIOMARKER
Predictive and pronounced response of Licaminlimab in patients with TNFR1-related biomarker highlights potential opportunity to transform the treatment paradigm of DED with a precision medicine approach.
Licaminlimab
an anti-TNFα eye drop candidate, could transform the treatment paradigm of DED with a precision medicine approach.
Find out more about Licaminlimab and its potential as the first anti-TNFα biologic eye drop to treat DED.