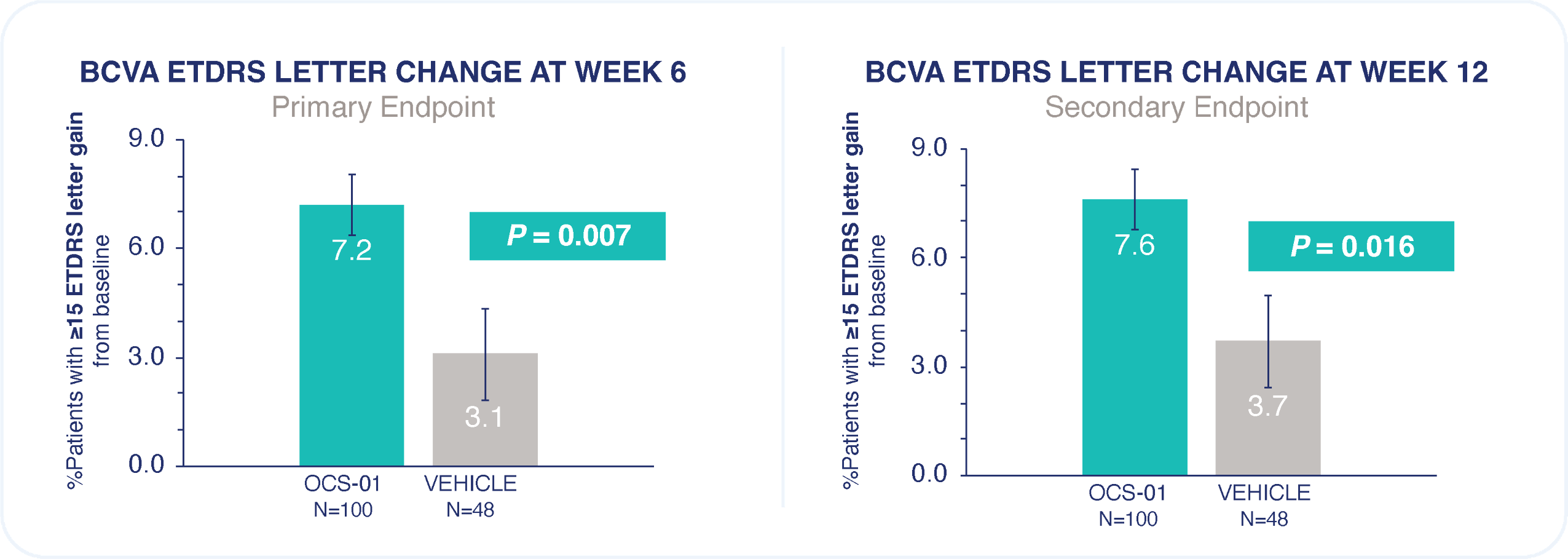
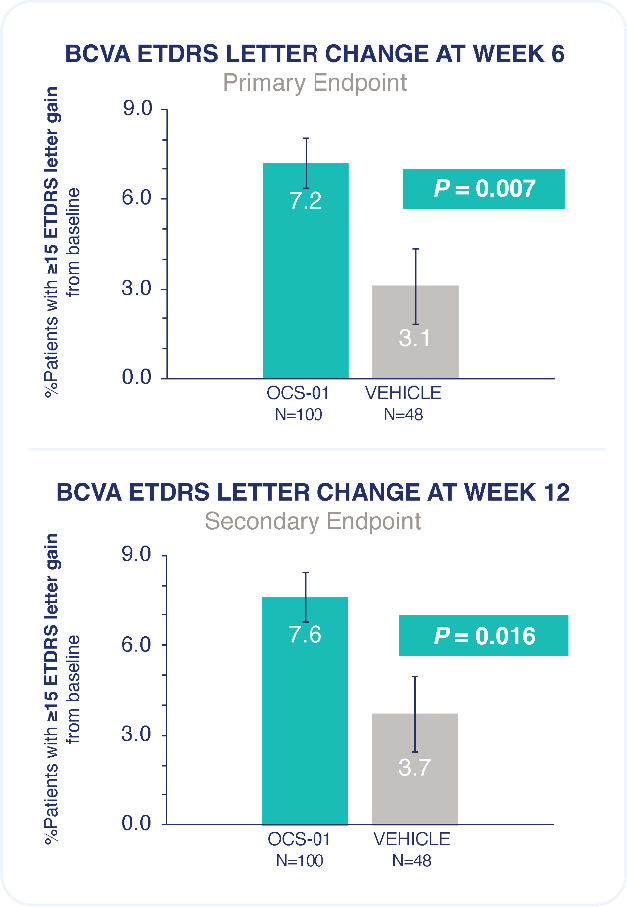
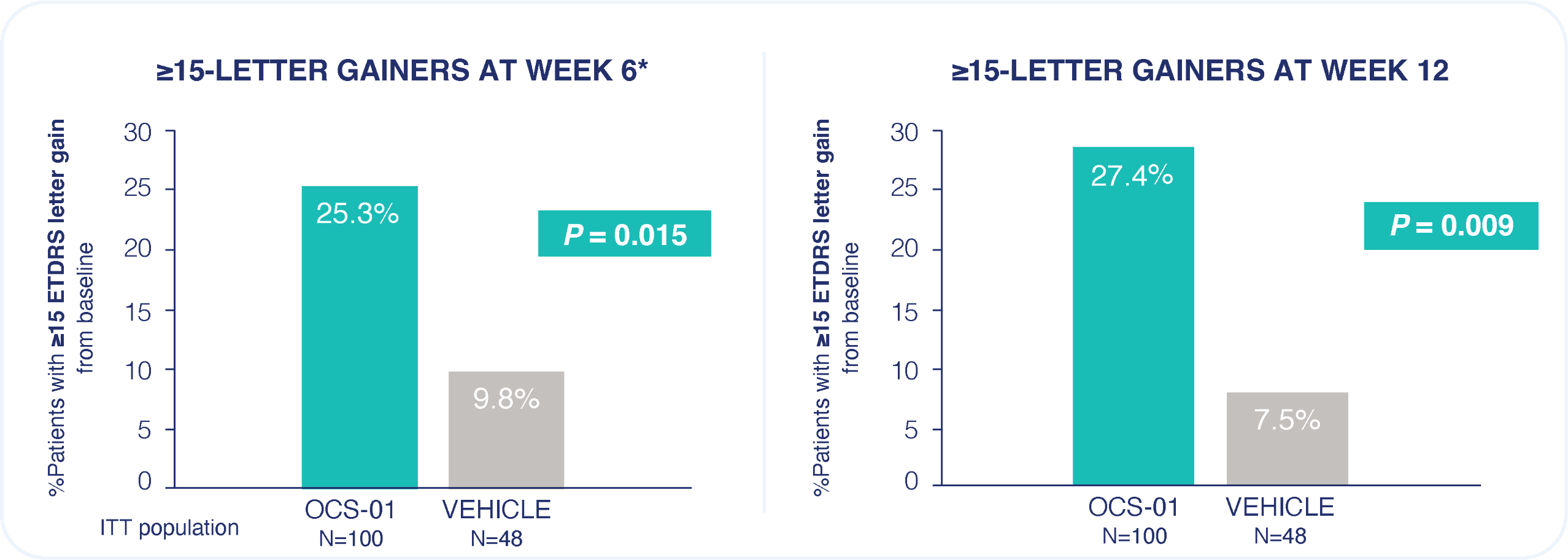
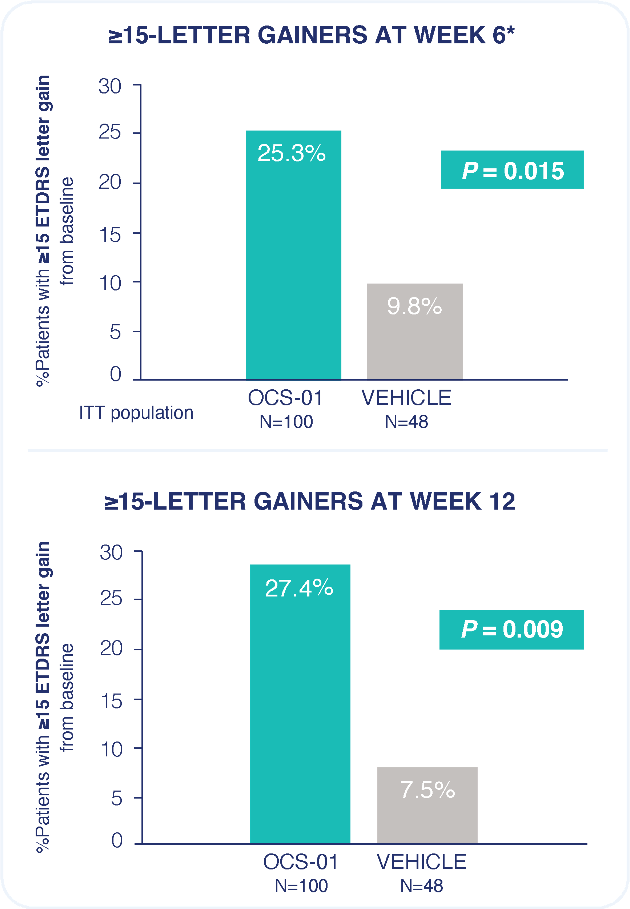
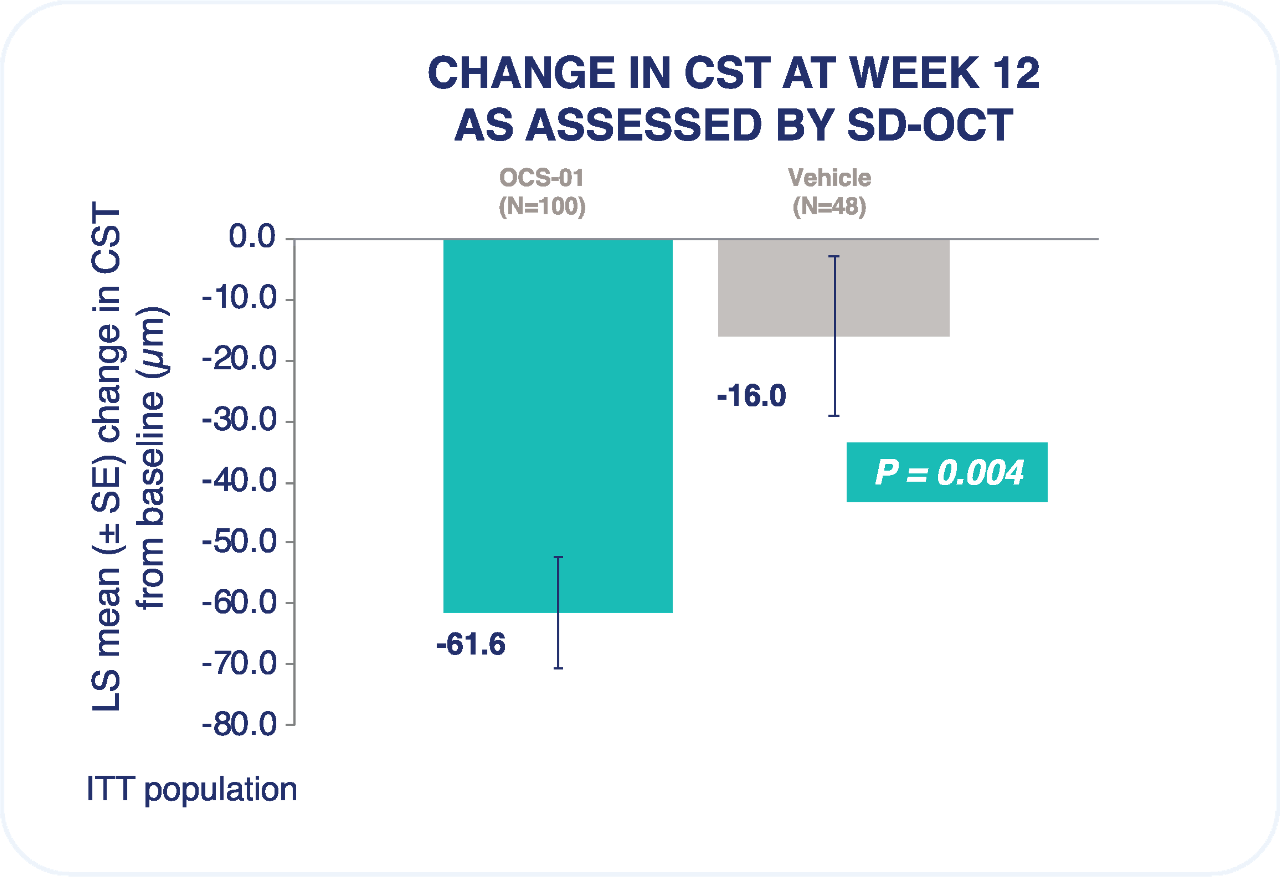
Rate of any ocular treatment-emergent adverse event was similar between OCS-01 and vehicle.
Stage 2 of the DIAMOND program was initiated in late 2023 following the positive results of Stage 1 and includes two 52-week parallel Phase 3 trials, DIAMOND-1 and DIAMOND-2.
OCS-01 is an investigational drug and has not received regulatory approval for commercial use in any country.