ACC: anterior chamber cells.
Data for visits after receipt of rescue medication, or missing data resulting from withdrawal due to adverse event or lack of efficacy, are singly imputed as failure. Missing data without withdrawal or resulting from withdrawal due to reasons other than adverse event or lack of efficacy are multiply imputed using treatment-based Markov Chain Monte Carlo methodology. Data, analysis, and conclusions are preliminary, and subject to change as full analysis is ongoing.
OCS-01 Phase 3 OPTIMIZE-1 Trial
Phase 3 Results of OCS-01 Eye Drops for the Treatment of Inflammation and Pain following Cataract Surgery


OCS-01 positive results highlight its potential to become the first once-daily, highly concentration, preservative-free steroid for treating inflammation and pain following ocular surgery.
OPTIMIZE-1 (Once-daily Post-ocular surgery Treatment for InflaMmation and paIn to minimiZE drops) is a double-blind, placebo-controlled Phase 3 trial conducted in 25 sites across the U.S. with 241 patients randomized 1:1 to receive once daily OCS-01 eye drop (n=119) or vehicle (n=122) for fourteen days following cataract surgery.
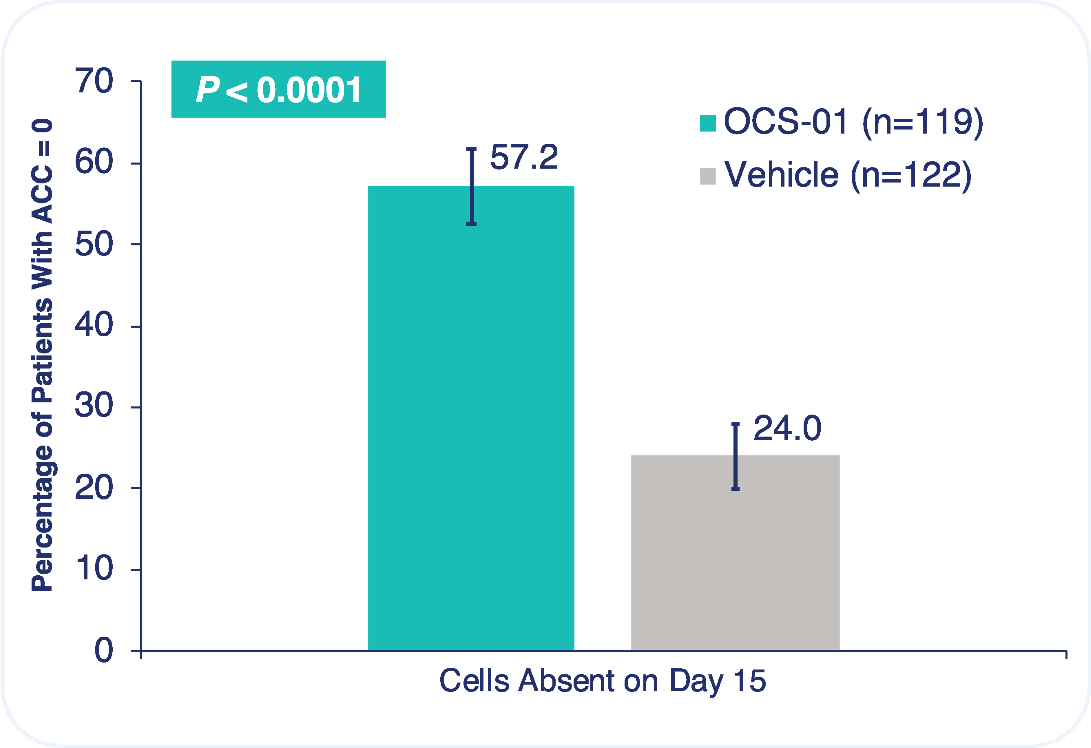
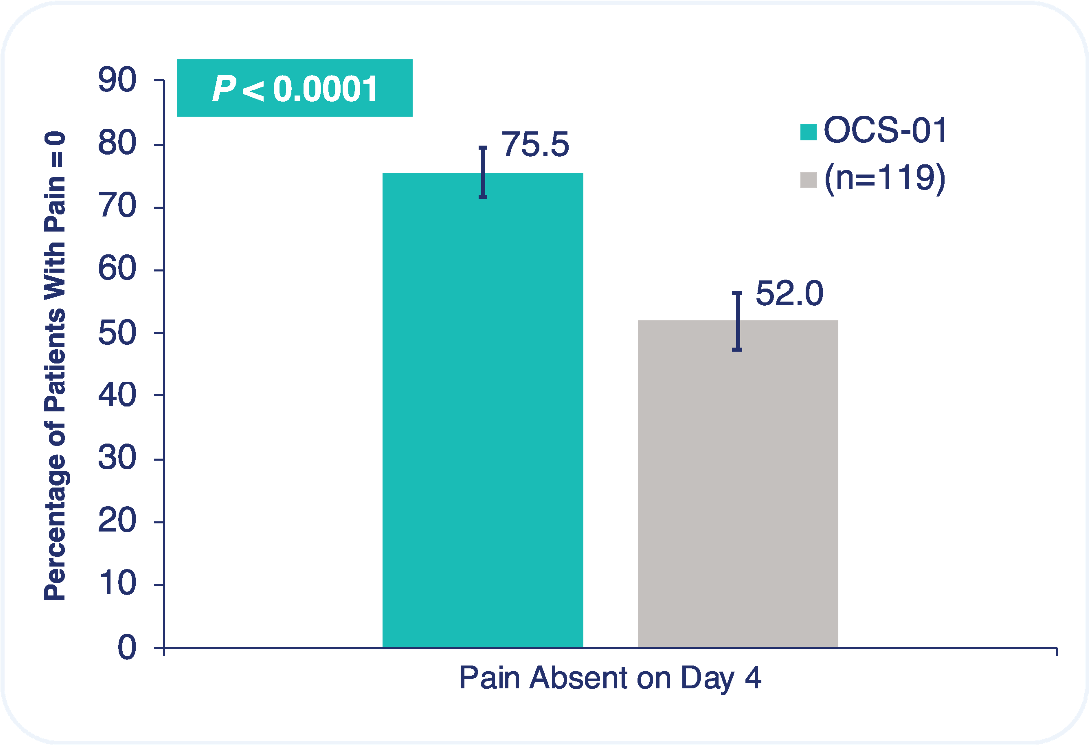
The trial met both primary efficacy endpoints, the absence of inflammation at Day 15 and the absence of pain at Day 4, with robust statistical significance.
Phase 3 OPTIMIZE-1 Trial Met Both Primary Endpoints with highly significant results in absence of inflammation and pain following cataract surgery with OCS-01 as compared to vehicle:
57.2% of patients had no inflammation at Day 15
75.5% of patients had no pain at Day 4
OPTIMIZE-1 (Once-daily Post-ocular surgery Treatment for InflaMmation and paIn to minimiZE drops) is a double-blind, placebo-controlled Phase 3 trial conducted in 25 sites across the U.S. with 241 patients randomized 1:1 to receive once daily OCS-01 eye drop (n=119) or vehicle (n=122) for fourteen days following cataract surgery.
The trial met both primary efficacy endpoints, the absence of inflammation at Day 15 and the absence of pain at Day 4, with robust statistical significance.
Phase 3 OPTIMIZE-1 Trial Met Both Primary Endpoints with highly significant results in absence of inflammation and pain following cataract surgery with OCS-01 as compared to vehicle:
57.2% of patients had no inflammation at Day 15
75.5% of patients had no pain at Day 4
Data for visits after receipt of rescue medication, or missing data resulting from withdrawal due to adverse event or lack of efficacy, are singly imputed as failure. Missing data without withdrawal or resulting from withdrawal due to reasons other than adverse event or lack of efficacy are multiply imputed using treatment-based Markov Chain Monte Carlo methodology. Data, analysis, and conclusions are preliminary, and subject to change as full analysis is ongoing.
Rethinking Ophthalmology to Save Sight and Improve Eye Care
Once daily OCS-01 could become an attractive option to treat pain and inflammation after ocular surgery with a highly potent anti-inflammatory effect. This could be especially beneficial for high-risk patients, such as diabetic patients, who face an increased risk of complications following ocular surgery due to pre-existing underlying inflammation.”