Optic Neuritis
Breakthrough Therapy Designation granted to Privosegtor for Treatment of Optic Neuritis
Privosegtor, a novel peptoid small molecule that penetrates blood brain and retinal barriers, has the potential to become the first neuroprotective therapy for optic neuropathies and other neuro-ophthalmic diseases


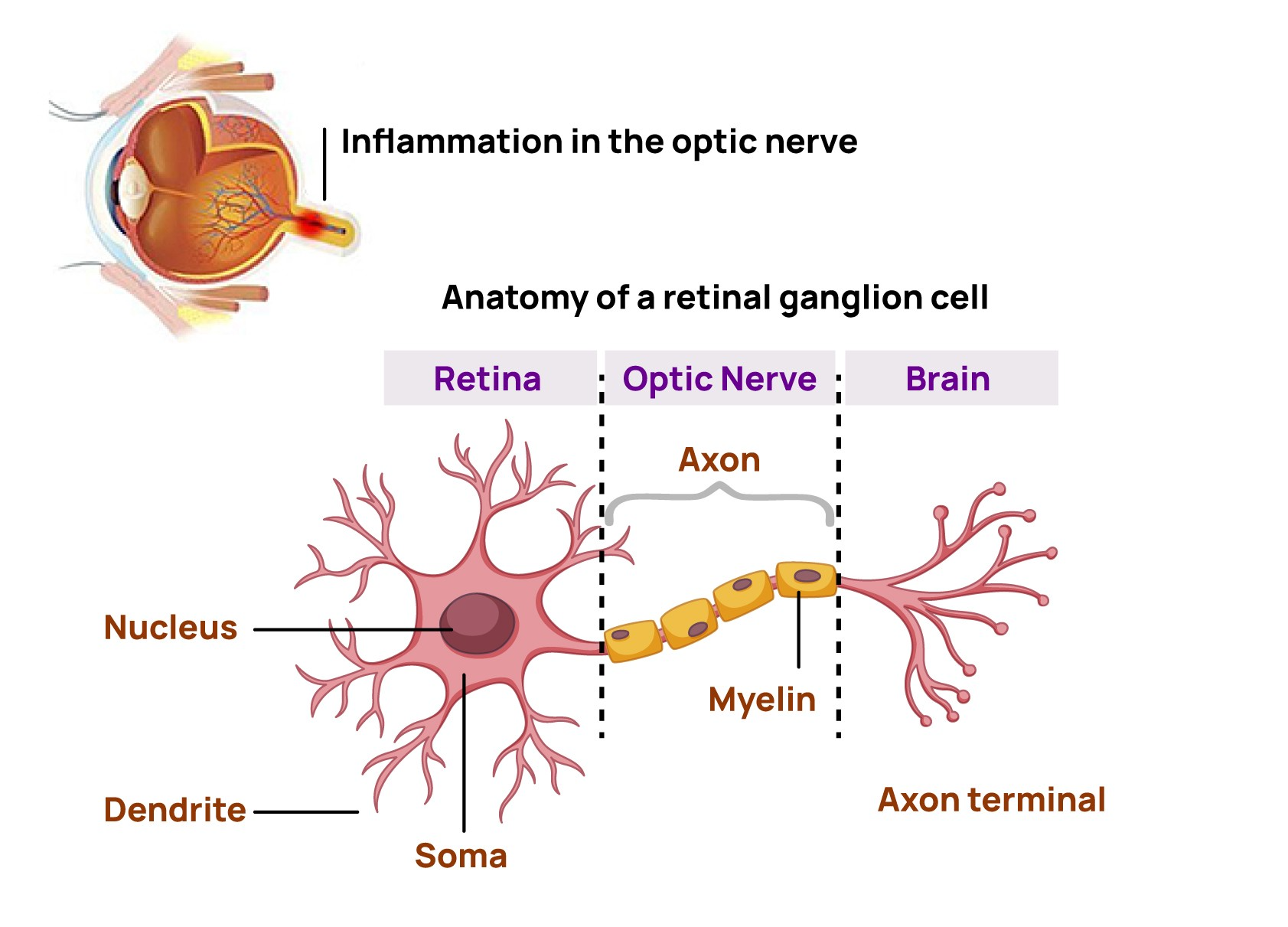
Optic neuritis is a condition characterized by an acute inflammation of the optic nerve that can lead to permanent visual impairment
Optic neuritis is when inflammation in your optic nerve causes pain, vision loss and other symptoms. This condition has strong links to chronic conditions like multiple sclerosis and other autoimmune diseases. Timely diagnosis and treatment may help optic neuritis and limit or delay more severe long-term effects or conditions.1
It is a type of neuropathy (nerve disease) and happens when inflammation affects signals traveling through your optic nerve, which connects your eyes and brain.
The cells that make up your optic nerve have a fatty coating called a myelin sheath. When you have optic neuritis, that sheath deteriorates. The coating is protective. Without it, the nerve cells can’t send signals properly. That’s why various forms of vision loss are common symptoms of this condition.1
To date there are no neuroprotective treatments available for optic neuritis and unmet needs remain for therapies that can prevent vision loss after an acute episode of optic neuritis by avoiding nerve cells damage or death.
Optic neuritis is a condition affecting up to 8 in 100,000 people worldwide2 per year and often represents the first sign of multiple sclerosis.
It mainly occurs in adults between the age of 20 and 40 years and is more frequent in women (2:1).
Unmet needs remain for neuroprotective therapies that can prevent vision loss after an acute episode of optic neuritis.

Privosegtor
a novel peptoid small molecule that penetrates the blood brain and retinal barriers, with the potential to become the first neuroprotective therapy for optic neuropathies and other neuro-ophthalmic diseases.
Privosegtor was selected by high-throughput screening (HTS) for neuroprotective properties, confirmed in vivo in glaucoma, multiple sclerosis, and optic neuritis models.
The data from in vitro studies suggest that it activates the serum–glucocorticoid kinase (SGK) and triggers multiple beneficial effects on apoptosis, oxidation, and inflammation.
Privosegtor has shown positive results in the prevention of retinal ganglion cell and axonal damage and was associated with improvements in clinical function (disability) in animal models of neuroinflammation and neurodegeneration.
In 2025, Oculis announced positive results from the ACUITY (Acute OptiC NeUrITis of DemYelinating Origin) trial, evaluating the safety, tolerability and efficacy of OCS-05 in patients with optic neuritis.
The trial met the primary endpoint of safety and achieved several key efficacy-based secondary endpoints, showing functional vision improvement and neuroprotective anatomical and biological benefits in patients suffering from an acute episode of optic neuritis.
Furthermore, the results of the study show that in addition to the standard of care (IV methylprednisolone), Privosegtor was tolerated in participants with optic neuritis. The most frequently reported drug-related treatment-emergent adverse events (TEAEs) in the Privosegtor (2 or 3 mg/kg/day) treatment group were headache and acne, each reported in 2 participants (10.5%).
Privosegtor has received Breakthrough Therapy designation from the FDA and Orphan Drug designation from both the FDA and the EMA for the treatment of optic neuritis.
Privosegtor is an investigational drug and its safety or efficacy has not been established and it has not received regulatory approval for commercial use in any country.
Privosegtor was selected by high-throughput screening (HTS) for neuroprotective properties, confirmed in vivo in glaucoma, multiple sclerosis, and optic neuritis models.
The data from in vitro studies suggest that it activates the serum–glucocorticoid kinase (SGK) and triggers multiple beneficial effects on apoptosis, oxidation, and inflammation.
Privosegtor has shown positive results in the prevention of retinal ganglion cell and axonal damage and was associated with improvements in clinical function (disability) in animal models of neuroinflammation and neurodegeneration.
In 2025, Oculis announced positive results from the ACUITY (Acute OptiC NeUrITis of DemYelinating Origin) trial, evaluating the safety, tolerability and efficacy of OCS-05 in patients with optic neuritis.
The trial met the primary endpoint of safety and achieved several key efficacy-based secondary endpoints, showing functional vision improvement and neuroprotective anatomical and biological benefits in patients suffering from an acute episode of optic neuritis.
Furthermore, the results of the study show that in addition to the standard of care (IV methylprednisolone), Privosegtor was tolerated in participants with optic neuritis. The most frequently reported drug-related treatment-emergent adverse events (TEAEs) in the Privosegtor (2 or 3 mg/kg/day) treatment group were headache and acne, each reported in 2 participants (10.5%).
Privosegtor has received Breakthrough Therapy designation from the FDA and Orphan Drug designation from both the FDA and the EMA for the treatment of optic neuritis.
Privosegtor is an investigational drug and its safety or efficacy has not been established and it has not received regulatory approval for commercial use in any country.
Explore Privosegtor Clinical Trials in Optic Neuritis

Registrational trial: Privosegtor in Patients with Optic Neuritis (PIONEER-1)
Registrational trial: Privosegtor in Patients with Optic Neuritis (PIONEER-2)
Visionary Innovation to Deliver Breakthrough Therapies
Optic neuritis is a disease characterized by acute inflammation and demyelination of the optic nerve. While corticosteroids are used to shorten the attack, there is no specific treatment approved for acute optic neuritis, and unmet needs remain for novel therapies that can prevent vision loss.